First published in MSF's International Activity Report 2015, available to download below.
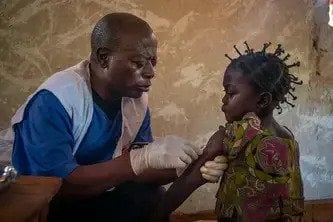
Carmen Jose-Panti is 36 years old. She lives with her husband and her two children in Tete, Mozambique. She runs a small business from home selling items such as soap and salt, and works in a health clinic where she manages patient files.
A mother, a wife, a business owner, an employee and a community member, Carmen is also HIV positive.
Having discovered she was infected with the virus in 2007, Carmen started antiretroviral (ARV) treatment in 2009.
Carmen is alive, healthy and able to contribute to her community because she takes generic versions of HIV medicines, ARVs made in India.
Carmen is one of more than 230,000 people worldwide living with HIV who receive treatment through MSF-supported HIV programmes. A recent survey of the drugs MSF buys for its field programmes shows that 97 per cent of those it uses to treat people living with HIV are generic medicines from India.
It’s not just ARVs that MSF turns to India for, though; more than three-quarters (77 per cent) of the drugs MSF uses to treat its 23,000 patients with tuberculosis (TB), and nearly a quarter (22 per cent) of the medicines it uses to treat 2.1 million cases of malaria are also generics from India.
India is known as the ‘pharmacy of the developing world’ for the volume of affordable essential medicines it supplies to developing countries – to people like Carmen.
How did India take on this role?
While the US, Switzerland and other high-income countries granted patent monopolies on medicines (meaning that companies had the sole right to make and sell them for a set period of time), India didn’t allow them until 2005, as it considered drugs to be an essential commodity requiring multiple suppliers and affordable prices.
Although international trade rules forced India to start granting product patents on medicines in 2005, it did so within stricter parameters – far tougher than those in wealthy and most developing countries – and also introduced other pro-public health provisions in the patent law that allowed for the continued supply of generic medicines already in production.
All this meant that the generics industry in India flourished and was able to produce medicines that were more affordable than the originals.
In 2001, ARVs to treat HIV were priced at over US$10,000 per patient per year. Millions of people living with the disease in Africa and other parts of the developing world died.
Indian generics company Cipla then started producing a single pill containing three drugs for just under US$1 per day.
Fifteen years later, nearly 16 million people – including Carmen – are receiving HIV treatment. Today, standard HIV treatment costs around US$100 per person per year.
Indian generics under threat
The laws and policies that enabled India to become the generics-producing powerhouse it is today are under increasing threat. Major pharmaceutical companies like Novartis first went on the attack in 2006.
After being denied a patent for a cancer drug in India, Swiss company Novartis legally challenged part of its patent law, which – if Novartis had won – would have had a dramatic impact on the country’s ability to produce generics, as it would have forced the Indian Patent Office to grant patents that were not merited under India’s public health and innovation-friendly law. The case lasted seven years and went all the way to India’s Supreme Court, but in April 2013, Novartis – to the relief of MSF and millions of people everywhere – lost the case and India’s law remained intact.
The EU is applying pressure on India in a different way, by pushing it to sign a free trade agreement that would severely curtail its production of generics. Negotiations started nine years ago and are ongoing.
In another agreement involving countries across south and southeast Asia – the Regional Comprehensive Economic Partnership – wealthy countries like Japan and South Korea are trying to introduce provisions that could block the trade in generic medicines between developing countries.
The greatest pressure has come from the US, however, with the US Trade Representative threatening India with trade sanctions if the government does not take steps to modify its patent law in line with the preferences of US drug companies, and grant more patents.
Over the years, Indian ministers, policy makers and government officials have withstood the pressure but now a new intellectual property policy drawn up by the
Indian government could signal a change in the way in which the patent law is interpreted and pharmaceutical patents are enforced. This policy could even eventually lead to legislative changes that would permanently undermine India’s status as the pharmacy of the developing world.
As MSF relies so heavily on generic medicines from India, it keeps a close eye on developments in the country through the Access Campaign. If the laws and policies change, newer generations of drugs won’t be available as generics, and MSF won’t be able to treat as many people.
New restrictions could also halt the production of paediatric versions and combinations of drugs to treat diseases like HIV – which make treatment for patients and the work of medical staff much easier. It would mean that in the future, our medical staff wouldn’t be able to provide the level of care that patients like Carmen deserve.
It’s for this reason that MSF continues to raise the alarm on this issue to make sure that India can remain open for business in the future.
In 2015, through the Access Campaign, MSF launched its ‘Hands Off Our Medicines’ campaign, urging India to stand strong amid the pressure to grant patents more widely and limit the production of affordable medicines.